Fluoride has long been the standard ingredient in anti-cavity toothpaste and is likely a component of your current daily oral care products. However, modern oral care brands are focusing on and seeking a scientifically supported alternative to fluoride. Hydroxyapatite is a very promising replacement to fluoride. Although currently you don’t find this ingredient in the formulations of most major pharmacy – famous branded toothpastes, hydroxyapatite has over 40 years of research and dental care application history to back it up. Moreover, hydroxyapatite is non toxic and biocompatible, making it a better option for those looking for natural oral care solutions.
Dr. Jonathan B. Levine, Chief Dental Officer of Twice, the oral health brand founded by Lenny Kravitz, said, “Hydroxyapatite (Hap) is the main component of tooth enamel and one of the most studied biomaterials in the medical and dental fields.” “Hydroxyapatite constitutes the main mineral part of bones and teeth, and in fact makes up more than 90% of the basis of tooth enamel.” As the outermost layer of teeth and the hardest substance in the human body, healthy tooth enamel is crucial for oral and overall health, as it protects the inside of the teeth from bacteria and tooth decay. When the enamel wears away and the dentin is exposed, bacteria and decay – causing organisms can lead to cavities, severe infections, gum disease, and even tooth loss. Hydroxyapatite has proved function in teeth remineralization.
The medical application of hydroxyapatite was first proposed by NASA in the 1970s, when astronauts lost minerals in their teeth and bones due to the lack of gravity in space. In 1974, Japan’s Miki Corporation obtained a patent from NASA for the use of hydroxyapatite in the dental field. Eventually, it produced the world’s first nano – hydroxyapatite toothpaste in 1980 and obtained a patent for this ingredient in 1990.
Levine said, “Hydroxyapatite does not have the controversial issue of excessive fluoride, and is considered safer in people’s minds.” Although dentists point out that fluoride is 100% safe to use because we don’t swallow toothpaste, and fluoride – containing toothpaste is necessary for people who need strong efficacy for weak enamel.
Hydroxyapatite can replace fluoride, but it is also safe to use with fluoride, and some toothpaste formulations combine these two ingredients. Dr. Chris Salierno, Chief Dental Officer of Tend, said, “Both hydroxyapatite and fluoride are minerals that can repair cavity damage.” Fluoride can kill bacteria, while hydroxyapatite can prevent bacteria from attaching to teeth.
The remineralization of tooth enamel is the main advantage of hydroxyapatite. By strengthening the enamel, teeth can better resist plaque, which can cause tooth decay and gum disease. Although fluoride can also prevent tooth decay, it does not contain calcium and phosphate. These minerals, which are present in hydroxyapatite and enamel, help prevent tooth decay. Dr. Chris Salierno explained, “Hydroxyapatite works behind the scenes to prevent cavities from forming or progressing.”
HAp toothpaste is effective for tooth sensitivity, and has the effects of preventing tooth decay and whitening teeth. Because hydroxyapatite naturally exists in our bodies, unlike fluoride, it is non – toxic and biocompatible. This also provides a safety guarantee for children who may accidentally swallow toothpaste.
Although you can’t see the benefits of hydroxyapatite – enamel is translucent, and Levine said “tooth remineralization occurs at the micrometer level” – you may feel them. Hydroxyapatite helps relieve tooth sensitivity by filling the aforementioned tubules, which are essentially microscopic pathways to the tooth nerves. The fewer exposed tubules, the less sensitivity and discomfort you feel to touch, food, and temperature.
Under normal toothpaste formulation and usage conditions, the possibility of a direct reaction between HAP and fluoride (e.g., sodium fluoride, NaF) to generate a large amount of calcium fluoride (CaF₂) is very low, and this is not the main reaction pathway. Due to the difference in solubility, HAP itself has extremely low solubility under neutral pH conditions. Sodium fluoride (NaF) dissolves to provide F⁻ ions. Although Ca²⁺ and F⁻ can combine to form CaF₂, this reaction requires free Ca²⁺ ions. Therefore, the compounding of HAP with fluoride (e.g., NaF) is a common and promising formulation strategy. The two can synergistically enhance the anti-caries and remineralization effects. As long as the pH of the formulation is properly controlled (to avoid being too low), it will not cause the inactivation of HAP or the production of harmful substances.
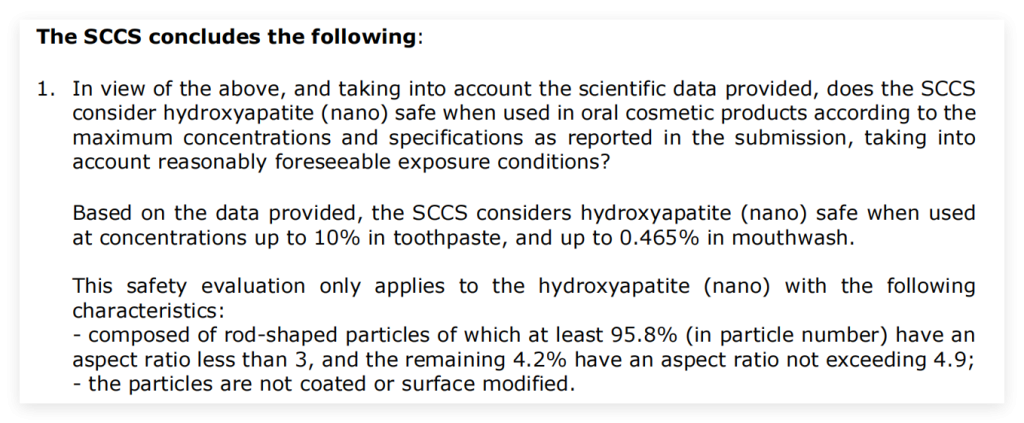
According to the opinion of the Scientific Committee on Consumer Safety (SCCS) regarding the application safety of nano hydroxyapatite, the SCCS considers nano hydroxyapatite safe when used at concentrations up to 10% in toothpaste, and up to 0.465% in mouthwash. However,this safety evaluation by SCCS only applies to the nano hydroxyapatite with aspect ratio<3.
Welcome to visit EPRUI’s Related Products:Nano Hydroxyapatite Powder for Toothpaste for more detailed information!