Project Description
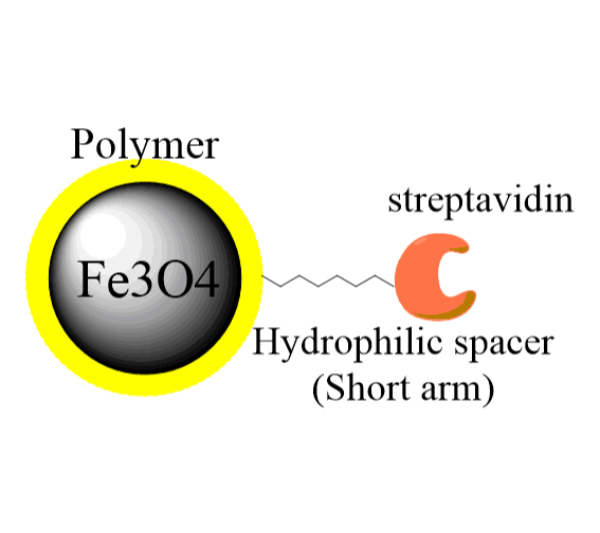
Streptavidin Magnetic Beads
Streptavidin magnetic beads or SA magnetic beads can universally bind to any biotinylated molecules, such as antibody, protein, peptide, DNA, etc., through high affinity interaction between streptavidin and biotin. The particles have a large surface area with high capture efficiencies.
The G-Streptavidin-biotin biomolecule complex is easy to be separated from unbound biotin-biomolecule using a magnet, therefore providing a quick and neat way to tag biomolecules with magnetic nanoparticles.
The purified nanoparticle-biomolecule complex can be used in a variety of downstream separation processes: protein purification, cell isolation, immunoprecipitation and molecular detection.
EPRUI Biotech Trusted By
Buying Guide of EPRUI’s Streptavidin Magnetic Beads
1. Streptavidin Magnetic Beads Specifications
| Item | APS | Concentration | Ligand |
|---|---|---|---|
| EPRUI-MagSA-02 | 200nm | 10mg/ml | Streptavidin |
| EPRUI-MagSA-1 | 1um | 10mg/ml | Streptavidin |
| EPRUI-MagSA-3 | 3um | 10mg/ml | Streptavidin |
| EPRUI-MagSA-5 | 5um | 10mg/ml | Streptavidin |
| EPRUI-MagSA-10 | 10um | 10mg/ml | Streptavidin |
| EPRUI-MagSA-20 | 20um | 10mg/ml | Streptavidin |
| EPRUI-MagSA-30 | 30um | 10mg/ml | Streptavidin |
| EPRUI-MagSA-40 | 40um | 10mg/ml | Streptavidin |
Binding Capacity:
- >50 μg Streptavidin/mg beads;
- >4000pmol free biotin/mg beads;
- >300pmolbiotin-oligonucleotide/mg beads;
- >20pmol biotin-dsDNA fragments/mg beads;
2. Why choose streptavidin magnetic beads from EPRUI?
· Guaranteed binding capacity
We have very strict quality control procedure: Each lot of our streptavidin magnetic beads is tested for the guaranteed minimum binding capacity with free biotin, which makes sure batch to batch reproducibility.
· Zero Non specific binding of proteins
2 mg SA magnetic particles are incubated with 0.2mg IgG in 200 ul 50mM phosphate buffer, 0.15 M NaCl, pH 7.2 for 1 h at 15 to 25 ℃. After washing with 2×200ul incubation buffer the protein concentration in the supernatant is measured (A280). Non specific binding is not detected.
· No DNase activity
1ug DNA substrate is incubated for 4h at 37℃ in 100ml buffer M(10mM tris-HCl, 10mM MgCl2, 50mM NaCl, 1mM dithioerythriol, pH7.5) with 1mg SA magnetic beads.DNase activity is not detected.
· No RNase activity
5ug MS2 RNA are incubated for 4h at 37℃ in 100ml 10mM tris-HCl, pH7.5 with 1mg SA magnetic particles. Ribonuclease activity is not detected.
· No protease activity
10mg SA beads are incubated with casein-resorufin in 200 ul 0.2M Tris-HCl, 20mM CaCl2, pH 7.8 for 30 min at 37℃. After TCA precipitation, the protease activity correlates with the absorbance at 574 nm in the supernatant. Protease activity is not detected.
3. Which brands can our streptavidin magnetic beads replace?
Streptavidin magnetic beads from EPRUI can replace almost all types of streptavidin magnetic beads in the market, such as GE sera mag ™ Magnetic Beads Streptavidin、Pierce Streptavidin Magnetic Beads、Dynabeads® MyOne ™ Streptavidin MS150 of JSR and shows the same or even better effect.
4. What’s the storage and delivery condition of streptavidin magnetic beads?
Storage condition: The streptavidin magnetic beads are store at 2-8°C(up to 6 months)in storage buffer (25 mM tris-HCl, 0.15 M NaCl, 0.05% tween20, 0.09% NaN3, pH 7.2). Do not freeze the reagent.
Delivery Condition: The streptavidin magnetic bead is packed in foam incubator with refrigerant ice pack.
5. Examples of specific applications of streptavidin magnetic beads
Example 1. Binding of Biotin-labeled Nucleic Acid to SA Magnetic Beads
This procedure can be used to bind biotinylated oligonucleotides and double stranded DNA.
- Vortex the Beads for 20 seconds before use.
- Pipet the required volume of PuriMag Beads into a nuclease-free microcentrifuge tube. Separate the magnetic particles for 30 seconds, using a magnetic separator. Note: 50 μL @ 10 mg/mL (500 μg) is sufficient to bind 125 pmol (~80 μg) biotinylatedoligonucleotide or a biotinylated PCR product (~ 40 μg @ 500 bp).
- Remove the supernatant. Resuspend beads in 100ul Binding Buffer (20 mM Tris·Cl, 1.0 M NaCl, 1 mM EDTA, 0.02% Triton® X-100; pH 7.8).
- Incubate appropriate amount of a biotinylated oligonucleotide with the resuspended the Beads for 15 mins at room temperature. Mix gently.
- Separate the Beads using a magnetic separator and wash the beads twice with an appropriate amount of Binding Buffer.
- Resuspend the beads in DEPC-treated water. The oligonucleotide-coated particles are now suitable for downstream application.
Example 2. Purification of mRNA from Total RNA
- Vortex the Beads for 20 seconds before use.
- Pipet required amount of beads into a nuclease-free tube. Magnetically separate beads.
- Remove the supernatant. Resuspend the beads in 100ul Binding Buffer (20 mM Tris·Cl, 0.5 M NaCl; pH 8.0).
- Incubate an appropriate amount of a 5′-biotinylated oligonucleotide (dT) with the resuspended beads from step 3 for 15 minutes at room temperature (15–25°C).
- Magnetically separate the beads. Discard the supernatant and wash the oligo-bound beads twice with 200 ul Binding Buffer.
- Add DEPC-treated water to the total RNA sample so that the final volume is 90 μl.
- Incubate total RNA sample at 55°C for 5 mins to disrupt secondary RNA structures.
- Add 10μl of 5M NaCl to total RNA sample.
- Add the total RNA to the washed magnetic beads from step 5. Mix gently and hybridize for 3 minutes at room temperature.
- Magnetically separate beads and discard the supernatant. Wash beads twice with 150ul Wash Buffer (7mM Tris·HCl, 0.17M NaCl; pH 8.0).
- Elute bound mRNA with appropriate amount of DEPC-treated water for 2 min at 55°C. Magnetically separate the beads and transfer the supernatant to a new tube.
Example3. Immobilization of Biotinylated Antibody
- Vortex the Beads for 20 seconds before use.
- Add 50μL (0.5mg) beads to 1 mL Binding Buffer (TBS-0.05% Tween 20 detergent). Wash beads twice with binding buffer.
- Resuspend particles in 450 ul Binding Buffer.
- Add 50 μl of serum or cell culture supernatant to the particles. Note: Modify sample volume according to user preference. If sample volume is <500μL, dilute it to a final volume of 500μL with Binding Buffer.
- Gently mix using rotator for 30 minutes.
- Magnetically separate the beads and discard supernatant. Wash beads twice with 0.5ml Binding Buffer to remove unbound proteins.
Example4. Cell Enrichment
EPRUI Streptavidin magnetic nanoparticles are ideal for isolation cells (e.g. CTCs, stem cells) from a mixture of cell population obtained from tissues or organs.
- Vortex the Beads for 20 seconds before use.
- Aliquot enough PuriMag Streptavidin Beads for enrichment experiment. Note: 20 μl is generally sufficient for the enrichment of up to 1×106 cells.
- Wash beads twice with 500μlWashing Buffer (TBS-0.05% Tween 20 detergent). Magnetically separate and remove the supernatant carefully.
- Add biotinylated antibody to the nanoparticle and incubate for 15-30 minutes using a sample rotator. Note: 20 μl nanoparticle could bind to 50-1000 ng antibody.
- Wash bead-antibody conjugates twice with 500μlWashing Buffer to remove unbound antibody.
- Resuspend the nanoparticle-antibody conjugates in Washing Buffer (20-50 μl) and add it to the cell sample to a total volume of 1-5 ml.
- Incubate the beads with the cell sample on an rotator for 30-60 minutes at room temperature.
- After incubation, use a magnet to separate the nanoparticles (with bound cells) from the solution, and carefully remove the supernatant.
- Wash beads with 500μlWashing Buffer twice.
- The isolated cells can be either kept on ice for immediate downstream applications or suspended in cell culture medium to grow.
6. How to order
Please send e-mail to: sales@epruibiotech.com or call us by 86-21-64192663 for products inquires.
For detailed steps, please visit: How to Buy


















